Download Lambert Beer Law Background
The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Let's take a closer look! The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .
Anda dapat berkonsultasi dengan konsultan hukum secara online via WhatsApp. Info VENDORHUKUM.COM
 Measuring transmittance and absorbance using Beer-Lambert's law... | Download Scientific Diagram from www.researchgate.net The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. Let's take a closer look!
Measuring transmittance and absorbance using Beer-Lambert's law... | Download Scientific Diagram from www.researchgate.net The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. Let's take a closer look!
Let's take a closer look! Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law.
7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . Let's take a closer look! The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .
 The Beer Lambert Law, also known as Lambert's Law, applied to Solar Energy Absorption by Water from img.bhs4.com Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Let's take a closer look! The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .
The Beer Lambert Law, also known as Lambert's Law, applied to Solar Energy Absorption by Water from img.bhs4.com Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Let's take a closer look! The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .
7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. Let's take a closer look!
The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . Let's take a closer look!
 PPT - Determining the Concentration of a Solution: Beer's Law PowerPoint Presentation - ID:5611981 from image3.slideserve.com The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. Let's take a closer look!
PPT - Determining the Concentration of a Solution: Beer's Law PowerPoint Presentation - ID:5611981 from image3.slideserve.com The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. Let's take a closer look!
Let's take a closer look! The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law.
Download Lambert Beer Law Background. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as . The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . Let's take a closer look! 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law.

7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .

Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when .

Let's take a closer look! This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law. The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as .
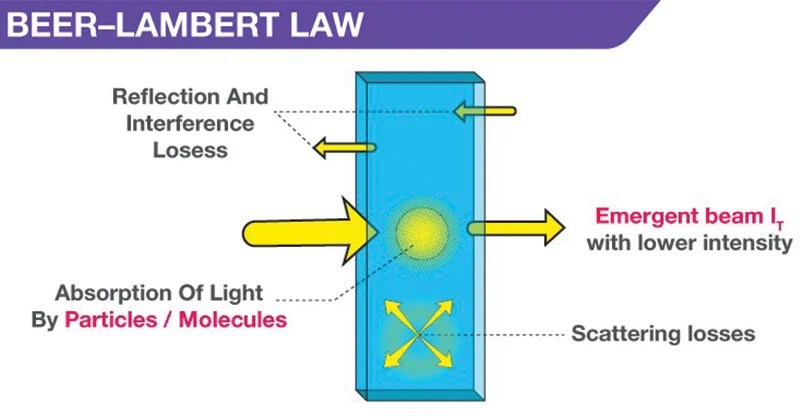
The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the . Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. Let's take a closer look!

Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer. 7.1.2 ultraviolet and visible light spectroscopy · when increasing the wavelength and decreasing the frequency of light, other effects are prone to happen when . The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .
This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law.

The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .

This technique is called spectrophotometry, and this type of analysis will involve calculations using beer's law.

Beer law states that concentration and absorbance are directly proportional to each other and it was stated by august beer.

The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the .
Kunjungi VENDORHUKUM.COM Untuk Konsultasi Masalah Hukum
0 Response to "Download Lambert Beer Law Background"
Post a Comment